B.A., San Francisco State University, 1974. Ph.D., California Institute of Technology, 1980 (Sunney Chan). Postdoctoral: University of California, Berkeley, 1980–83 (Randy Schekman). Honors and Awards: Damon Runyon-Walter Winchell Postdoctoral Fellow, 1980–82; American Cancer Society Faculty Research Award, 1988–93; Medical Research Foundation of Oregon Discovery Award, 1997; Elected Fellow, American Academy of Microbiology, 1998; College of Arts and Sciences Distinguished Professor, 2005. Philip H. Knight Professorship, 2007. American Association for the Advancement of Science Fellow, 2013. At Oregon since 1983.
Research in the Stevens lab is concerned with the process of protein sorting and membrane assembly in yeast cells. Using yeast molecular genetics, we have identified a large number of genes required for the correct targeting and transport of proteins to the membrane-bounded organelle called the vacuole. These vacuolar protein sorting (VPS) genes have been found to encode proteins such as a dynamin-like GTPase, a protein-sorting receptor, a protein kinase, a lipid kinase, a RAS inhibitor-like protein, and an increasingly large number of proteins involved in transport vesicle targeting/fusion such as Rab-like GTPases and SNARE proteins. To characterize the function of some of these proteins we use biochemical, cell biological and molecular genetic approaches. Biochemical approaches are being used to isolate a number of the VPS proteins and to study the membrane-associated protein complexes in which they are found.
Figure 1
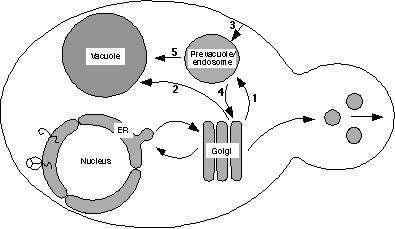
Schematic drawing of the yeast endomembrane pathways. Proteins exiting the Golgi complex can be transported by either the CPY pathway (arrow 1) through the prevacuole to the vacuole, or by the ALP pathway (arrow 2). Proteins can also reach the prevacuole by endocytosis (arrow 3), and proteins in the prevacuole transit a common pathway to the vacuole (arrow 5). Golgi membrane proteins such as DPAP A are retrieved from the prevacuole back to the Golgi (arrow 4).
The group also has a long-standing interest in the assembly, targeting, structure and function of the vacuolar H+-translocating ATPase (V-ATPase; see figure). The V-ATPase complex consists of fourteen subunits, and all but one of these are encoded by a single yeast gene. The large hydrophobic V-ATPase “a” subunit has two isoforms, Vph1 and Stv1, with the Vph1-associated V-ATPase complex localizing to the vacuole membrane and the Stv1-associated V-ATPase restricted to Golgi and endosomal membranes. The mechanism of differential localization of these two forms of the yeast V-ATPase is under active investigation in the lab. We are investigating the proteins responsible for maintaining this differential localization, as well as the protein-based signals that specify the distinct localizations.
We have also identified five genes that encode proteins required for V-ATPase complex assembly but are not themselves part of the final V-ATPase enzyme complex. These five proteins reside in the yeast cell endoplasmic reticulum and constitute the dedicated assembly machinery for the V-ATPase. A number of molecular genetic and biochemical approaches are being taken to characterize the assembly complex and to study the interaction of this assembly complex with V-ATPase subunits along the assembly pathway within the endoplasmic reticulum.
Figure 2
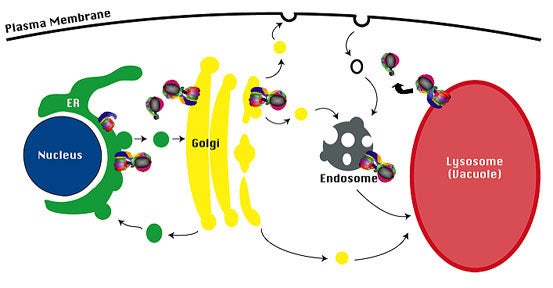
Targeting of V-ATPase complexes.The membrane sector of the V-ATPase complex (V 0 sector) is assembled in the ER, and the assembled V-ATPase complex is then transported to the Golgi complex. The Stv1-containing V-ATPase complex cycles between the endosome and the Golgi complex, and the Vph1-containing V-ATPase complex is transported to the limiting vacuole membrane.
The lab has also employed ancestral gene reconstruction to investigate the V-ATPase enzyme complex in more detail. Ancestral reconstruction of the two V-ATPase subunit a isoforms has generated the most likely predecessor gene prior to gene duplication. This Anc.a protein functions with the remaining 13 V-ATPase subunits and has characteristics of both the Stv1- and Vph1-containing V-ATPase complexes. Ancestral reconstruction of the most likely predecessor of the Vma3 and Vma11 proteolipid V-ATPase subunits has led to the synthesis of an Anc.3-11 the functions with the remaining 12 V-ATPase subunits to form a functional enzyme complex. The Anc.3-11 protein allows the assembly of a proteolipid ring with two different polypeptides (Anc.3-11 and Vma16) rather than the modern-day ring with three different polypeptides (Vma3, Vma11 & Vma16). These investigations have revealed important insights into the modern-day V-ATPase complex as well as the evolution of this V-ATPase molecular machine.
Figure 3
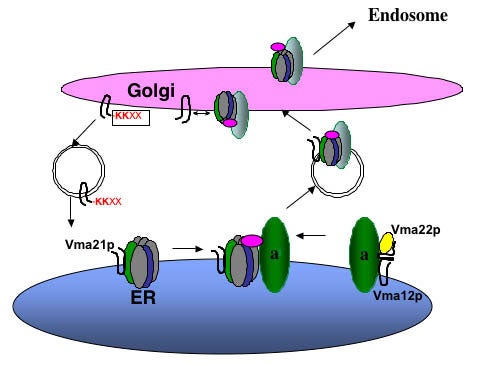
Assembly of the Vo sector in the ER. Vma21p interacts with the proteolipid ring V-ATPase subunits of Vma3p (c), Vma11p (c’), and Vma16p (c’’). Vma12p and Vma22p form a complex on the ER membrane and interact with newly synthesized “a” subunit (Vph1p and Stv1p), and then Vma21p “escorts” this Vo sector into vesicles budding from the ER membrane. Vma21p accompanies the V-ATPase to the Golgi complex, where its C-terminal KKXX ER retention signal is recognized by the COPI machinery and this assembly/escort factor is recycled to the ER. The assembled V-ATPase is then transported on to the endosome and/or vacuole.
Finnigan, G.C.*, Hanson-Smith, V.*, Stevens, T.H. and J.W. Thornton (2012) The evolution of a multi-paralog molecular machine. Nature, Jan 9, 2012 online.
Finnigan, G.C.*, Hanson-Smith, V.*, Houser, B.D., Park, H.J. and T.H. Stevens (2011) The reconstructed ancestral subunit a functions as both V-ATPase isoforms Vph1p and Stv1p in S. cerevisiae. Mol. Biol. Cell, 22:3176-3191.
Finnigan, G.C., Ryan, M. and T.H. Stevens (2011) A genome-wide enhancer screen implicates sphingolipid composition in vacuolar ATPase function in Saccharomyces cerevisiae. Genetics, 187:771-783.
Coonrod, E.M. and T.H. Stevens (2010) The Yeast vps Class E Mutants: The Beginning of the Molecular Analysis of the Multivesicular Body Biogenesis. Mol. Biol. Cell, 21:4057-4060.
Ryan, M., Graham, L.A. and T.H. Stevens (2008) Voa1p functions in V-ATPase membrane sector assembly in the yeast endoplasmic reticulum. Mol. Biol. Cell, 19:5131-5142.
Flannery, A.R. and T.H. Stevens (2008) Functional characterization of the N-terminal domain of Subunit H (Vma13p) of the Yeast Vacuolar ATPase. J. Biol. Chem., 283:29099-29108.
Neubert, C., Graham, L.A., Black-Maier, E.W., Liu, T.-Y., Stierhof, Y.-D., Seidel, T., Stevens, T.H. and K. Schumacher (2008) Arabidopsis has two functional orthologs of the yeast V-ATPase assembly factor Vma21p. Traffic, 9:1618-1628.
Schluter, C., Lam, K.K.Y., Brumm, J., Wu, B.W., Saunders, M., Stevens, T.H., Bryan, J. and E. Conibear (2008) Global analysis of yeast endosomal transport identifies the Vps55/68 sorting complex. Mol. Biol. Cell, 19:1282-1294.
Davis-Kaplan, S.R., Compton, M.A., Flannery, A.R., Ward, D.M., Kaplan, J., Stevens, T.H. and L.A. Graham (2006) PKR1 encodes an assembly factor for the yeast V-type ATPase. J Biol Chem. 281: 32025-32035.
Compton, M.A., Graham, L.A. and T.H. Stevens (2006) Vma9p (subunit e) is an integral membrane V0 subunit of the yeast V-ATPase. J Biol Chem. 281:15312-15319.
Lottridge, J.M., Flannery, A.R., Vincelli, J.L. and T.H. Stevens (2006) Vta1p and Vps46p regulate the membrane association and ATPase activity of Vps4p at the yeast multivesicular body. Proc Natl Acad Sci U S A. 103:6202-6207.
Bowers, K. and T.H. Stevens. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta, 1744, 438-454 (2005).
Malkus, P., Graham, L.A., Stevens, T.H. and R. Schekman. Role of Vma21p in assembly and transport of the yeast vacuolar ATPase. Mol. Biol. Cell, 15, 5075-5091 (2004).
Flannery, A.R., Graham, L.A. and T.H. Stevens. Topological characterization of the c, c', and c'' subunits of the Vacuolar ATPase from the yeast Saccharomyces cerevisiae. J. Biol. Chem., 279, 39856-39862 (2004).
Bowers, K., Lottridge, J., Helliwell, S.B., Goldthwaite, L.M., Luzio, J.P. and T.H. Stevens. Protein-protein interactions of ESCRT complexes in the yeast Saccharomyces cerevisiae. Traffic, 5, 194-210 (2004).
Graham, L.A., Flannery, A.R. and T.H. Stevens. Structure and assembly of the yeast V-ATPase. J. Bioenerg. Biomembr., 35, 301-312 (2003).
Conibear, E., Cleck, J.N. and T.H. Stevens. Vps51p mediates the association of the GARP (Vps52/53/54) complex with the Golgi t-SNARE Tlg1p. Mol. Biol. Cell, 14, 1610-1623 (2003).
Bowers, K. and T.H. Stevens. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta, 1744, 438-454 (2005).
Malkus, P., Graham, L.A., Stevens, T.H. and R. Schekman. Role of Vma21p in assembly and transport of the yeast vacuolar ATPase. Mol. Biol. Cell, 15, 5075-5091 (2004).
Flannery, A.R., Graham, L.A. and T.H. Stevens. Topological characterization of the c, c', and c'' subunits of the Vacuolar ATPase from the yeast Saccharomyces cerevisiae. J. Biol. Chem., 279, 39856-39862 (2004).
Bowers, K., Lottridge, J., Helliwell, S.B., Goldthwaite, L.M., Luzio, J.P. and T.H. Stevens. Protein-protein interactions of ESCRT complexes in the yeast Saccharomyces cerevisiae. Traffic, 5, 194-210 (2004).
Graham, L.A., Bowers, K., Flannery, A.R. and T.H. Stevens. The role of the V-ATPase in the cellular physiology of the yeast Saccharomyces Cerevisiae. Handbook of ATPases: Biochemistry, Cell Biology, Pathophysiology, Futai, Kaplan & Wada eds., 355-377 (2004).
Bowman, E.J., Graham, L.A., Stevens, T.H. and B.J. Bowman. The bafilomycin/concanamycin binding site in subunit c of the V-ATPases from Neurospora crassa and Saccharomyces cerevisiae. J. Biol. Chem., 279, 33131-33138 (2004).
Graham, L.A., Flannery, A.R. and T.H. Stevens. Structure and assembly of the yeast V-ATPase. J. Bioenerg. Biomembr., 35, 301-312 (2003).
Conibear, E., Cleck, J.N. and T.H. Stevens. Vps51p mediates the association of the GARP (Vps52/53/54) complex with the Golgi t-SNARE Tlg1p. Mol. Biol. Cell, 14, 1610-1623 (2003).
Kweon, Y., Rothe, A., Conibear, E. and T.H. Stevens. Ykt6p is a multifunctional yeast R-SNARE that is required for multiple membrane transport pathways to the vacuole. Mol. Biol. Cell, 14, 1868-1881 (2003).
Conibear, E. and T.H. Stevens. Studying yeast vacuoles. Methods Enzymol., 351, 408-432 (2002).
Kawasaki-Nishi, S., Bowers, K., Nishi, T., Forgac, M. and T.H. Stevens. The amino-terminal domain of the V-ATPase a subunit controls targeting and in vivo dissociation and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J. Biol. Chem., 276, 47411-47120 (2001).
Sagermann, M., Stevens, T.H. and B.W. Matthews. Crystal structure of the regulatory subunit H of the V-type ATPase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA, 98, 7134-7139 (2001).
Bowers, K., Levi, B.P., Patel, F.I. and T.H. Stevens. The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell, 11, 4277-4294 (2000).
Powell, B., Graham, L.A. and T.H. Stevens. Molecular characterization of the yeast vacuolar H+-ATPase proton pore. J. Biol. Chem., 275, 23654-23660 (2000).
Gerrard, S.R., Levi, B.P. and T.H. Stevens. Pep12p is a multifunctional yeast syntaxin that controls entry of biosynthetic endocytic and retrograde traffic into the prevacuolar compartment. Traffic, 1, 259-269 (2000).
Gerrard, S.R., Bryant, N.J. and T.H. Stevens. Vps21 controls entry of endocytosed and biosynthetic proteins into the yeast prevacuolar compartment. Mol. Biol. Cell, 11, 613-626 (2000).
Conibear E. and T.H. Stevens. Vps52p, Vps53p and Vps54p forms a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol. Biol. Cell 11, 305-323 (2000).
Graham, L.A., Powell, B. and T.H. Stevens. Composition and assembly of the yeast vacuolar H+-ATPase complex. J. Exp. Biol. 203, 61-70 (2000).
Gerrard, S.R., Mecklem, A.B. and T.H. Stevens. The yeast endosomal t-SNARE, Pep12p, functions in the absence of its transmembrane domain. Traffic 1, 45-55 (2000).
Tishgarten, T., Yin, F.F., Faucher, K., Dluhy, R., Fischer von Mollard, G., Stevens, T.H. and L.A. Lipscomb. Novel structures and oligomerization states for yeast v- and t-SNAREs. Prot. Sci. 8, 2465-2473 (1999).
Ungermann, C., Fischer von Mollard, G., Jensen, O.N., Margolis, N., Stevens, T.H. and W. Wickner. Three v-SNAREs and two t-SNAREs are essential for homotypic vacuole fusion. J. Cell Biol. 145, 1435-1442 (1999).
Zheng, H., Fischer von Mollard, G., Kovaleva, V., Stevens, T.H. and N.V. Raikhel. The Plant v-SNARE AtVTI1a Likely Mediates Vesicle Transport from the TGN to the Prevacuole. Mol. Biol. Cell 10, 2251-2264 (1999)
Fischer von Mollard, G. and T.H. Stevens. TheSaccharomyces cerevisiae: v-SNARE Vti1p is required for multiple transport pathways to the vacuole. Mol. Biol. Cell 10, 1719-1732 (1999).
Graham, L.A. and T.H. Stevens. Assembly of the yeast vacuolar proton-translocating ATPase. J. Bioener. and Biomem. 31, 39-47 (1999).
Conibear, E. and T.H. Stevens (1998) Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta 1404: 211-230.
Bryant, N.J., Piper, R.C., Weisman, L.S. and T.H. Stevens (1998) Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J. Cell Biol. 142: 651-663.
Graham, L.A., Hill, K.J. and T.H. Stevens (1998) Assembly of the yeast vacuolar H+-ATPase occurs in the endoplasmic reticulum and requires a Vma12p/Vma22p assembly complex. J. Cell Biol. 142: 39-49.
Bryant, N.J., Piper, R.C., Gerrard, S.R. and T.H. Stevens (1998) Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45-independent, endocytic route. Eur. J. Cell Biol. 76: 43-52.
Fischer von Mollard, G. and T.H. Stevens (1998) A human homologue can functionally replace the yeast v-SNARE Vti1p in two vesicle transport pathways. J. Biol. Chem. 273: 2624-2630.
Voos, W. and T.H. Stevens (1998) Retrieval of resident late-Golgi membrane proteins from the prevacuolar compartment of Saccharomyces cerevisiae is dependent on the function of Grd19p. J. Cell Biol. 140: 577-590.
Bryant, N.J. and T.H. Stevens (1998) Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol. and Molec. Biol. Rev. 230-247.
Jackson, D.D. and T.H. Stevens (1997) VMA12 encodes a yeast endoplasmic reticulum protein required for vacuolar H+-ATPase assembly. J. Biol. Chem. 272: 25928-25934.
Tomashek, J.J., Graham, L.A., Hutchins, M.U., Stevens, T.H. and D.J. Klionsky (1997) V1-situated stalk subunits of the yeast vacuolar proton-translocating ATPase. J. Biol. Chem. 272: 26787-26793.
Stevens, T.H. and M. Forgac (1997) Structure, function and regulation of the vacuolar H+-ATPases. Ann. Rev. Cell & Devel. Biol. 13: 779-808.
Piper, R.C., Bryant, N.J. and T.H. Stevens (1997) The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J. Cell Biol. 138: 531-545.
Fischer von Mollard, G., Nothwehr, S.F. and T.H. Stevens (1997) The yeast v-SNARE mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J. Cell Biol. 137: 1511-1524.
Tellam, J.T., James, D.E., Stevens, T.H. and R.C. Piper (1997) Identification of a mammalian Golgi Sec1p-like protein; mVps45p. J. Biol. Chem. 272: 6187-6193.
Bryant, N.J. and T.H. Stevens (1997) Two separate signals act independently to localize a yeast late-Golgi membrane protein through a combination of retrieval and static retention. J. Cell Biol. 136: 287-297.
Hirata, R., Graham, L.A., Takatsuki, A., Stevens, T.H. and Y. Anraku (1997) VMA11 and VMA16 encode the second and third proteolipid subunits of the Saccharomyces cerevisiae vacuolar membrane H+-ATPase. J. Biol. Chem. 272: 4795-4803.
Cooper, A.A. and T.H. Stevens (1996) Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133: 529-541.
Nothwehr, S.N., Bryant, N.J. and T.H. Stevens (1996) The newly identified yeast GRD genes are required for retention of late-Golgi membrane proteins. Mol. Cell. Biol. 16: 2700-2707.
Chen, Y.-J. and T.H. Stevens (1996) The VPS8 gene is required for localization and trafficking of the CPY sorting receptor in Saccharomyces cerevisiae. Eur. J. Cell Biol. 70: 289-297.
Ekena, K. and T.H. Stevens (1995) The Saccharomyces cerevisiae MVP1 gene interacts with VPS1 and is required for vacuolar protein sorting. Mol. Cell. Biol. 15: 1671-1678.
Nothwehr, S.F., Conibear, E. and T.H. Stevens (1995) Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J. Cell Biol. 129: 35-46.
Hill, K.J. and T.H. Stevens (1995) Vma22p is a novel endoplasmic reticulum-associated protein required for assembly of the yeast vacuolar H+-ATPase Complex. J. Biol. Chem. 270: 22329-22336.
Piper, R.C., Cooper, A.A., Yang, H. and T.H. Stevens (1995) Vps27p controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 131: 603-617.
Hill, K. J., and T. H. Stevens (1994) Vma21p is a yeast membrane protein retained in the endoplasmic reticulum by a di-lysine motif and is required for the assembly of the vacuolar H+-ATPase Complex. Mol. Biol. Cell 5: 1039-1050.
Piper, R. C., E. A. Whitters, and T. H. Stevens (1994) Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur. J. Cell Biol. 65: 305-318.

